Island Pharmaceuticals Strengthens Galidesivir Position with New US Filoviridae Patent - An Antiviral Solution for your Watchlist.
- Noel Ong

- Jan 20
- 7 min read
Announcement
US Patent Granted for Galidesivir in filoviridae viruses – 7 January 2026 (view the announcement)
The Island Pharmaceutical story is another new one for the Samso News platform as we look to expand our coverage in the health sector. The business of keeping human beings healthy and monitoring for potential medical issues is probably one of the best form on revenue generation. Recently, I saw a post on LinkedIn where someone mentioned that the monitoring for ovarian cancer does not exist.
As humanity struggles with the need to maintain a longer life, it is often forgotten that the obstacles required to make that a reality. In terms of ASX businesses, I am now understanding why the high-risk biotech sector exists and continues to attract risk capital. Since establishing Samso News in 2025, we have been covering several companies that we feel are worth some good old DYOR.

Island Pharmaceuticals Limited (ASX: ILA) is advancing its antiviral strategy through the clinical development of Galidesivir, a broad-spectrum antiviral targeting high-consequence viral threats, including Marburg and Ebola. The program is positioned within a global biosecurity and public health framework, with regulatory engagement centred on the United States Food and Drug Administration (FDA) and potential inclusion in government stockpiles.
The Business of Island Pharmaceuticals: Galidesvir Antiviral Programs
Island Pharmaceuticals is focused on two clinical-stage antiviral assets (Figure 1):

Figure 1: Two clinical-stage antiviral assets (source: ILA)
Galidesivir – A broad-spectrum antiviral with activity demonstrated (Figure 2) across more than 20 RNA viruses, including Category A biothreats such as Marburg and Ebola, supported by extensive non-human primate (NHP) efficacy data and Phase 1 human safety studies.

Figure 2: Broad-spectrum antiviral with activity demonstrated (source: ILA)
ISLA-101 – A repurposed small-molecule antiviral targeting dengue and other mosquito-borne diseases, with a well-established human safety profile and completed Phase 2a/b dengue trial.
The Company’s strategy is underpinned by regulatory pathways that prioritise speed to approval for unmet medical needs, including the FDA’s Animal Rule.
How Island Pharmaceuticals Galidesivir Antiviral Strategy Works
Galidesivir (also known as BCX4430) is a broad-spectrum antiviral designed to stop viruses from replicating once they enter human cells.
The core mechanism:
Galidesivir tricks the virus during replication.
Many dangerous viruses (like flaviviruses and filoviruses) copy themselves using an enzyme called RNA-dependent RNA polymerase (RdRp).
Galidesivir is a nucleoside analogue—a synthetic molecule that closely resembles one of the natural RNA building blocks.
When the virus tries to copy its RNA, Galidesivir gets inserted into the growing viral RNA chain instead of the correct building block.
Once inserted, it halts or fatally disrupts RNA synthesis, preventing the virus from completing replication.
No replication → no spread inside the body.
Why this matters
Broad-spectrum: Because many RNA viruses rely on RdRp, Galidesivir has activity against multiple viruses rather than just one.
Early-stage intervention: It’s most effective when given early, before viral load escalates.
Host-independent: It targets a viral process, not human DNA or RNA, reducing off-target toxicity risk.
Viruses studied with Galidesivir
Research has shown activity against:
Yellow fever
Zika
Dengue
Ebola
Marburg
Other RNA viruses with conserved replication machinery
Highlights (7 January 2026): US Patent Grant Strengthens Galidesivir IP Position
US Patent No. 12,508,266 granted, covering the use of Galidesivir for the treatment of filoviridae viruses, including Marburg and Ebola.
Patent protection extends through October 2031, materially strengthening the commercial and strategic framework of the Galidesivir program.
The patent aligns directly with Island’s clinical focus on Marburg, following confirmation of the FDA Animal Rule pathway for approval in November 2025.
The approval represents another successful conversion within the Galidesivir patent portfolio acquired by Island, reinforcing ongoing execution of its IP expansion strategy.
Leadership Commentary
CEO and Managing Director, Dr David Foster, commented:
“This latest patent grant highlights that our IP footprint continues to go from strength to strength, and further validates the vigour of the patent portfolio that we gained full ownership rights of as part of the Galidesivir acquisition.”
“What is particularly pleasing about this patent is that it provides IP protection over a broad range of claims in the application of Galidesivir to treat filoviruses, in direct alignment with detailed clinical development pathway for the treatment of Marburg – a member of the filoviridae virus family.”
“As we continue to advance the study design in close consultation with the FDA, this patent approval provides a strong framework to further expand our IP footprint alongside our clinical progress.”
About the Project: Galidesivir Program
Galidesivir is a clinical-stage, small-molecule antiviral with demonstrated activity against more than 20 RNA viruses across multiple virus families, including filoviridae, flaviviridae, and coronaviridae.
Historical non-human primate studies showed:
94% overall survival in Marburg-infected primates, with 100% survival when dosed up to 48 hours post-infection.
100% survival in Ebola models (Figure 3) when Galidesivir was administered immediately or within 48 hours post-infection, with reduced but meaningful survival at later dosing intervals.
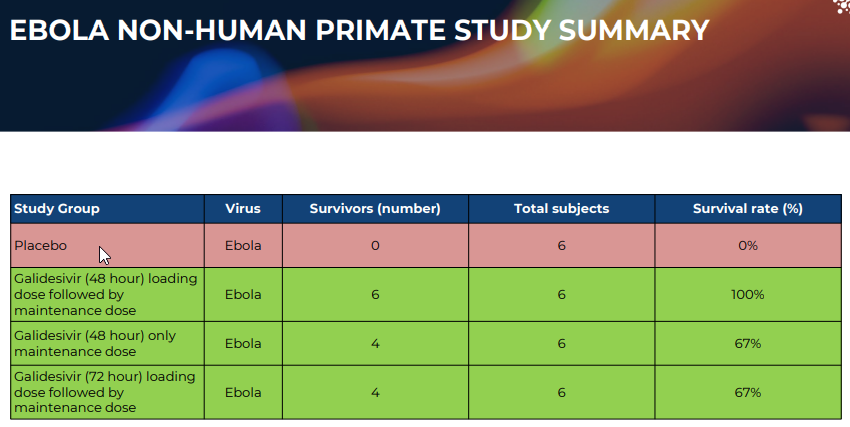
Figure 3: Ebola Non-Human Primate Study Summary” (source: ILA)
These results form the basis of Island’s regulatory engagement under the FDA Animal Rule (Figure 4).

Figure 4: FDA Animal Rule: A Proven Regulatory Pathway with Significant Strategic and Commercial Precedent (source: ILA)
Near-term Milestones to Watch
(Figure 5)
Preparation of the New Drug Application (NDA) for Galidesivir under the FDA Animal Rule – Q1 CY2026.
Filing of the NDA with the US FDA for approval of Galidesivir as a treatment for Marburg viral infection – Q2 CY2026.
Continued expansion of the Galidesivir patent portfolio alongside regulatory progression.

Figure 5: Near-term Milestones – Galidesivir (source: ILA)
Samso Concluding Comments
As discussed in the beginning of this blog, our association with the company business is still early. What we have learnt is that Island Pharmaceuticals continues to methodically build a defensible antiviral platform anchored by Galidesivir. The combination of clinical data, regulatory alignment, and now strengthened IP coverage reflects hard work and less headline-driven momentum.
The 7 January 2026 patent grant adds tangible depth to the Galidesivir story. Intellectual property remains the main purpose when engaging with regulators, partners, and government procurement agencies.
Market Implication
From an investment strategic perspective, ILA is just starting to move in terms of share price (Figure 6) and is in the process of revenue generation. I feel that is reasonably placed at AUD $123.70M that may be reflecting more about the stage the business is at, rather than its value - that is the way I see it in my equity valuation thinking.
The valuation of all companies that are publicly listed is beholden to the moods of the market. As valuation is simply the number of shares multiplied by the price, it is easily able to move up or down via the market fluctuations. Does a share price drop (other than a material reason) in a market sell off a reflection of the individual prospects?

Figure 6: ILA share price charts as of 15th January 2026. (source: CommSec)
For me, the journey for Samso News to look at the ins and outs of Island Pharmaceuticals will be exciting to share with the Samso community. For now, as we all say, DYOR.
The Samso Way – Seek the Research
Here at Samso, we pride ourselves on delivering content for investors that is independent and informed by over three decades of experience in the industry. Our content is well-researched and is only created if I see merit in discussing the company's story.
Our mission is simple: cut through the noise and spotlight what matters—genuine stories, grounded insights, and real opportunity.
Our content is well-researched and is only created if the team sees merit in discussing the company or concept. Investors can explore our three core platforms:
There may be numerous paths to success in investing, but the common thread among successful individuals is that they remain committed to making informed decisions. Equip yourself with the right knowledge and tools, and you will be well on your way to achieving your financial goals.
Most importantly, investors need to be absolutely diligent in understanding their own risk-reward tolerance and capabilities. Never bite off more than you can chew. As they say, Rome wasn’t built in a day, and the Great Wall stood because it took centuries to complete.
The Samso Philosophy:
Stay curious. Stay sharp. And remember—digging deeper always uncovers the real value.
In Life, there is no such thing as a Free Lunch.
Never bite off more than you can chew is my parting comment.
Happy Investing, and the only four-letter word you need to know is DYOR.
To support our independent nature of our work, please head over to our Support Page and give us a helping hand in any of the ways listed. This is a new initiate for the Samso Platform, and it was always the concept of Samso when we started this journey in 2018.
Disclaimer
The information or opinions provided herein do not constitute investment advice, an offer, or solicitation to subscribe for, purchase, or sell the investment product(s) mentioned herein. It does not take into consideration, nor have any regard to your specific investment objectives, financial situation, risk profile, tax position and particular, or unique needs and constraints.
Share to Grow: Your Bonus
Samso has just released an eBook: How to Add Value to your Share Portfolio
A lesson on geological models sought by mining companies that gives insight and an understanding of which portfolios are better - and potentially more lucrative – investments. Click here to download this eBook.
If you find this article informative and useful, please help me share the information. I try to write about topics that are interesting and have the potential to be of investment value. It is not easy to find stories that fit those parameters. If you or your organisation sees the benefit of what Samso is trying to achieve and has a need to share your journey, please contact me at noel.ong@samso.com.au.
Samso is a trusted platform that equips dedicated investors with up-to-date industry knowledge and insights from top CEOs and thought leaders. By staying informed on business advancements and market trends, investors can enhance their financial decisions through a combination of expert guidance and their own research.





Comments