Actinogen Medical Limited (ASX:ACW) Reaches Critical Milestone with 100th Patient in XanaMIA Alzheimer’s Trial.
- Noel Ong

- Aug 7, 2025
- 7 min read
Announcement

Actinogen Medical Ltd (ASX: ACW) has announced a significant milestone in the development of its lead candidate, Xanamem® (emestedastat), with the 100th participant now enrolled in its pivotal XanaMIA Phase 2b/3 Alzheimer’s disease trial. This event formally triggers the timeline for an independent interim analysis of safety and efficacy, bringing the company one step closer to potentially introducing a novel oral therapy for Alzheimer’s disease (AD).
Actinogen Chief Executive Officer, Dr Steven Gourlay, commented:
“We are delighted to confirm the timeline for our independent Data Monitoring Committee review of safety and efficacy data based on the enrolment of the 100th patient in the XanaMIA phase 2b/3 Alzheimer’s trial. This confidential review of unblinded data will ensure the XanaMIA trial is on-track and that Xanamem continues to perform as a promising and well-tolerated, once-a-day oral therapy.”
“Furthermore, with our 35 Australian and US clinical sites now enrolling at full pace, we are able to reconfirm our guidance for final results in late 2026. The availability of a follow-on, open-label trial where all will receive active Xanamem therapy should give participants and their families more incentive to participate in the current XanaMIA trial as well as provide valuable long-term safety and efficacy data.”
Key Highlights – XanaMIA Alzheimer’s Disease Phase 2b/3Trial.
100th patient enrolled and cleared for treatment – now scheduled for dosing in July 2025.
Interim data readout expected January 2026, based on the 24-week visit of this cohort.
Final results anticipated for Q4 2026 for the full 220-patient cohort.
35 active clinical sites operating across Australia and the US, with enrolment progressing steadily.
Independent Data Monitoring Committee (DMC) will assess unblinded data for both safety and efficacy futility.
Open-label extension trial (XanaMIA-DUR) planned to commence Q1 2026, offering Xanamem to all prior participants.
The XanaMIA trial is designed to rigorously assess the safety and efficacy of Xanamem 10 mg once daily in patients with mild to moderate Alzheimer’s disease and biomarker-confirmed progressive disease. The trial's primary efficacy measure is the CDR-SB (Clinical Dementia Rating Scale – Sum of Boxes), widely accepted as a robust clinical endpoint.
Clinical & Regulatory Pathway: A Structured Build Toward Approval.
The independent DMC, comprising experienced clinicians and statisticians, will conduct a confidential review of unblinded data, meaning the allocation of participants to active or placebo arms will only be known to the committee. Their task is to assess whether continuing the trial is futile based on efficacy trends or if any safety concerns warrant early termination. It is important to note that the committee cannot recommend early cessation due to success, only due to futility or safety.
Actinogen plans to engage with the US FDA in H2 2025 to discuss potential regulatory pathways, including expedited options, should the interim results exceed expectations. As a base case, Actinogen is preparing for a conventional regulatory approval process for Xanamem as a daily oral therapy for Alzheimer’s.
The company is also preparing for its second pivotal Phase 3 trial, expected to commence in Q1 2027, potentially in partnership with a commercial collaborator to support the next development stage and future launch.
Xanamem: A Distinct Therapeutic Approach.
Xanamem is a first-in-class, brain-penetrant inhibitor of the 11β-HSD1 enzyme, designed to regulate cortisol levels within the brain without impacting systemic cortisol production (Figure 1). Chronically elevated cortisol has been implicated in neurodegeneration and cognitive decline, making it a validated and increasingly targeted mechanism in both Alzheimer’s and depression research.
Oral Xanamem® (emestedastat)
Controlling brain cortisol to slow progression in Alzheimer’s disease
and treat depression.
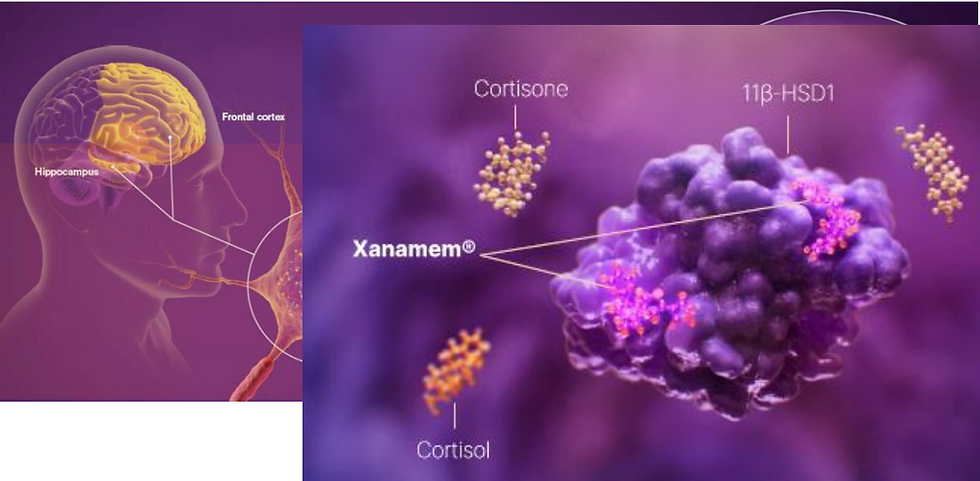
Figure 1: Xanamem - a small molecule tissue cortisol synthesis inhibitor (11β-HSD1 enzyme) (source: ACW)
The trial design capitalises on this biology. Xanamem has already shown statistically significant improvements in depressive symptoms (Figure 2) in its Phase 2a depression trial (XanaCIDD), and encouraging signals of slowed cognitive decline in Alzheimer’s patients with elevated pTau181 biomarkers (Figure 3).

Figure 2: Xanamem controls cortisol by inhibition of 11β-HSD1 (source: ACW)

Figure 3: Large Xanamem benefit in high pTau181 patients (source: ACW)
These results support the disease-modifying potential of Xanamem, a particularly attractive feature in an Alzheimer’s market still seeking oral, safe, and scalable treatment options (Figure 4).

Figure 4: XanaMIA phase 2b/3 trial in Alzheimer’s disease (source: ACW)
Well-Funded into 2026
The progress in the clinic is supported by a solid financial foundation. As disclosed on 30 June 2025, Actinogen secured a non-dilutive R&D tax funding package of up to $13.8 million, with $3.0 million already received and a further $10.8 million conditionally committed pending regulatory approvals. Combined with $13.4 million in existing cash, the company’s total funds sit at an estimated $16.4 million, providing a runway to at least mid-2026.
Actinogen Chief Financial Officer, Mr Will Souter, commented:
“The non-dilutive funding package from Endpoints, a firm with deep industry expertise, is an endorsement of the robustness of our program. Endpoints conducted rigorous due diligence to assess the veracity and likely eligibility of Actinogen’s anticipated FY25 RDTI before approving the loan. The funding further strengthens our balance sheet as we progress towards important milestones for the Company, including the upcoming interim analysis for our Alzheimer’s phase 2b/3 clinical trial, and reaffirms our cash runway out to mid-2026.”
This funding is earmarked to support both the XanaMIA trial and general operations, reinforcing Actinogen’s capacity to deliver on multiple clinical and regulatory milestones in the coming 12–18 months.
Samso Concluding Comments
Actinogen Medical continues to quietly but confidently advance a unique, mechanism-driven candidate in one of the most challenging and high-value areas of medicine—Alzheimer’s disease. From someone who is not from the industry, I am thinking that the 100th patient milestone may mean more than just signify progress; a hundred participants should give some form of clarity, via an independent DMC review, on whether Xanamem has genuine potential to alter disease trajectory in a scalable, convenient format.
I guess the broader context matters. With antibody drugs dominating headlines despite invasive delivery, cost, and modest benefit, an effective once-daily oral treatment—especially one with an add-on profile—could shift the therapeutic landscape. This trial design, paired with the validated pTau181 biomarker approach, gives Actinogen a clear differentiation angle.
Xanamem’s mechanism—targeting brain cortisol through 11β-HSD1 inhibition—remains novel and backed by peer-reviewed data in both Alzheimer’s and depression. The drug’s oral, once-daily profile positions it as a practical alternative in a market where current treatments often come with complexity and cost. If efficacy trends continue in the right direction, Xanamem could fill a gap the market still hasn’t addressed.
For those tracking value progression in biotech, this is the stage where the risk profile begins to shift. The 100-patient interim review won’t answer everything, but it will offer the first real test of Xanamem’s clinical potential. The pieces are in place—now it’s about what the data reveals. Investors looking for fundamentals, not froth, should be paying close attention.
The Samso Way – Seek the Research
Reaching the 100th participant in the XanaMIA trial is a pivotal milestone that shifts Actinogen’s Alzheimer’s program from potential to tangible progress. It marks the beginning of formal, independent scrutiny through the upcoming January 2026 interim analysis—a critical checkpoint that will assess whether Xanamem is on track or not. For investors, this is where real clinical validation begins. The decision by the Data Monitoring Committee will provide clarity on futility and shape confidence in the path ahead. Coupled with non-dilutive funding, FDA engagement, and a disciplined trial strategy, this moment signals that Actinogen is entering a decisive phase in its journey toward regulatory relevance and potential therapeutic impact.
Our mission is simple: cut through the noise and spotlight what matters—genuine stories, grounded insights, and real opportunity.
Our content is well-researched and is only created if the team sees a merit in discussing the company or concept. Investors can explore our three core platforms:
There may be numerous paths to success in investing, but the common thread among successful individuals is that they remain committed to making informed decisions. Equip yourself with the right knowledge and tools, and you will be well on your way to achieving your financial goals.
Most importantly, investors need to be absolutely diligent in understanding their own risk-reward tolerance and capabilities. Never bite off more than you can chew. As they say, Rome wasn’t built in a day, and the Great Wall stood because it took centuries to complete.
The Samso Philosophy:
Stay curious. Stay sharp. And remember—digging deeper always uncovers the real value.
In Life, there is no such thing as a Free Lunch.
Happy Investing, and the only four-letter word you need to know is DYOR.
To support our independent nature of our work, please head over to our Support Page and give us a helping hand in any of the ways listed. This is a new initiate for the Samso Platform, and it was always the concept of Samso when we started this journey in 2018.
Disclaimer
The information or opinions provided herein do not constitute investment advice, an offer or solicitation to subscribe for, purchase or sell the investment product(s) mentioned herein. It does not take into consideration, nor have any regard to your specific investment objectives, financial situation, risk profile, tax position and particular, or unique needs and constraints.
Share to Grow: Your Bonus
Samso has just released an eBook: How to Add Value to your Share Portfolio
A lesson on geological models sought by mining companies that gives insight and an understanding of which portfolios are better - and potentially more lucrative – investments. Click here to download this eBook.
If you find this article informative and useful, please help me share the information. I try and write about topics that are interesting and have the potential to be of investment value. It is not easy to find stories that fit those parameters. If you or your organisation see the benefit of what Samso is trying to achieve and have a need to share your journey, please contact me at noel.ong@samso.com.au.
Samso is a trusted platform that equips dedicated investors with up-to-date industry knowledge and insights from top CEOs and thought leaders. By staying informed on business advancements and market trends, investors can enhance their financial decisions through a combination of expert guidance and their own research.





Comments