Alterity Therapeutics (ASX: ATH) Advances Neurodegenerative Drug Development - Multiple system Atrophy.
- Noel Ong

- Aug 8, 2025
- 7 min read
Announcement

Alterity Therapeutics Limited (ASX: ATH; NASDAQ: ATHE) has announced highly promising topline results from its open-label Phase 2 clinical trial (ATH434-202) evaluating ATH434 in patients with advanced Multiple System Atrophy (MSA). The findings reinforce the company’s position as a front-runner in developing disease-modifying therapies for neurodegenerative diseases.
ATH434 has previously shown positive outcomes in a double-blind Phase 2 trial. This open-label trial in a more advanced patient group confirmed a similar level of clinical benefit. Importantly, the study showed that ATH434 slowed progression on both clinical and biomarker endpoints, was well-tolerated, and demonstrated target engagement in affected brain regions.
What is Multiple System Atrophy (MSA)?
Multiple system atrophy (MSA) is a progressive neurodegenerative disorder characterized by a combination of symptoms that affect both the central nervous system (which controls how a person moves), and the autonomic nervous system, which controls involuntary functions such as blood pressure or digestion. MSA was formerly known as Shy-Drager syndrome, olivopontocerebellar atrophy (OCPA), or striatonigral degeneration.
The symptoms of MSA reflect the progressive loss of function and death of different types of nerve cells in the brain and spinal cord. MSA is one of a family of neurological disorders known as an atypical parkinsonian disorder. The initial symptoms can be difficult to distinguish from those of Parkinson's disease, and can include:
Slowness of movement, tremor, or stiffness
Clumsiness or lack of coordination
Croaky, quivering voice
Fainting or lightheadedness
Bladder control problems
Symptoms tend to appear in a person's 50s and advance rapidly over the course of five to 10 years. A person with MSA will have increased difficulty with movement and eventually become bedridden. People with MSA often develop swallowing problems that can lead to pneumonia in the later stages of the disease.
There are two different types of MSA, which are categorized by the person’s most prominent symptoms when they’re evaluated by a doctor:
Parkinsonian type MSA (MSA-P) has primary symptoms similar to Parkinson's disease (such as slowness of movement, stiffness, and tremor) along with problems with balance, coordination, and autonomic nervous system dysfunction (such as urinary problems, sweating abnormalities, and digestion difficulties).
Cerebellar type MSA (MSA-C) is associated with balance and coordination problems (ataxia), difficulty swallowing, speech problems or a quivering voice, and abnormal eye movements.
MSA tends to progress more rapidly than Parkinson's disease, and most people with MSA will require an aid for walking, such as a cane or walker, within a few years after symptoms begin.
Key Highlights & Summary of Positive Topline Data from Open-Label Phase 2 Clinical Trial of ATH434 in MSA.
1. Clinical Benefit Observed
ATH434 reduced disease progression, with UMSARS I scores increasing by only 3.5 points over 12 months, compared to 6.5 in historical controls.
43% of patients completing the study had stable UMSARS scores.
30% of participants stabilized or improved in both Clinical and Patient Global Impression of Change scores.
Stabilisation of orthostatic hypotension symptoms was also observed.
2. Robust Biomarker Signals
Brain Volume: Mean z-score of brain volume change was -0.44, indicating slowed brain atrophy in MSA-affected regions.
Brain Iron Accumulation: Reduced iron buildup in the globus pallidus and putamen confirmed drug-target engagement.
NFL Levels: Neurofilament light chain levels in plasma and CSF remained stable, further supporting disease modification.
3. Safety Profile
ATH434 was well-tolerated. No serious adverse events related to treatment were reported.
Most side effects were mild to moderate in severity.
No negative impact on hemoglobin or iron parameters was observed.
4. Consistent with Earlier Study
ATH434-202 results mirror those of the earlier ATH434-201 double-blind trial, despite involving a more advanced MSA population.
Reinforces the therapeutic potential of ATH434 across a broader disease spectrum.
David Stamler, M.D., Chief Executive Officer of Alterity, commented:
“I am very encouraged by the positive results from the ATH434-202 trial, as they reinforce the robust efficacy we observed in our double-blind study,” said David Stamler, M.D., Chief Executive Officer of Alterity. “
M.D., M.S., Professor of Neurology at Vanderbilt University Medical Center and
principal investigator for the ATH434-202 Phase 2 study, commented:
“These results are very helpful in establishing the clinical response to therapy. The consistent changes in UMSARS, along with quantitative measures in imaging, support the findings we noted in Study 201. Currently, there are no disease modifying medications for the treatment of MSA, and these data encourage the continued development of ATH434 to treat this disease. We are indebted to the study participants and their families who contributed to this study.”
About ATH434 and Multiple System Atrophy
ATH434 is an oral iron chaperone, an oral therapeutic targeting pathological protein aggregation in neurodegenerative disorders. It acts as an iron chaperone, redistributing excess iron in the brain, one of the key pathological mechanisms in MSA (Figure 1).
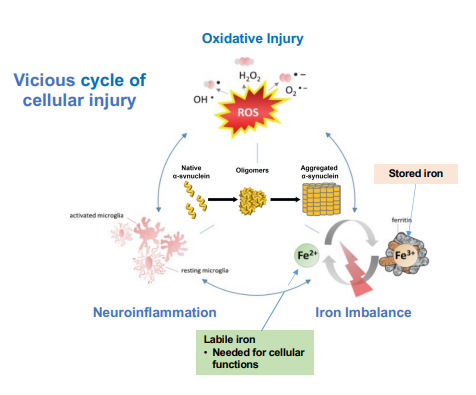
Figure 1: Excess labile iron and alpha-synuclein aggregation driving MSA pathology (Source: Alterity Phase 2 Presentation at MSA Symposium 2025)
MSA is a rapidly progressing and rare Parkinsonian disorder with no approved disease-modifying therapies. It affects the autonomic nervous system and motor control, with hallmark α-synuclein protein accumulation in glial cells.
ATH434 has received:
Fast Track Designation (FDA)
Orphan Drug Designation (FDA & European Commission)
Alterity continues its commitment to progressing ATH434 and alleviating the burden of MSA, with ongoing Phase 2 trials and additional pipeline development in related neurodegenerative conditions.
Samso Concluding Comments
This announcement from Alterity Therapeutics is confirming the business and the data is underpinning the clarity. In a disease like Multiple System Atrophy, where options are scarce and the clinical landscape is filled with uncertainty, a stabilisation of symptoms—let alone evidence of slowing disease progression—is no small achievement.
The consistency between the open-label and double-blind Phase 2 trials of ATH434 is particularly important. It speaks to the reproducibility of the treatment effect, which is a major hurdle in neurodegenerative research. From a clinical development perspective, this is the kind of validation that builds confidence in the path forward. From an investor's point of view, it places Alterity on firmer ground as it moves towards larger-scale studies and potential regulatory engagement.
There’s also something quite compelling about the mechanistic angle, targeting iron dysregulation and α-synuclein aggregation. This isn’t just symptomatic relief. It’s an effort to interfere directly with disease biology, backed by biomarker data showing measurable changes in brain structure and chemistry.
While biotech is never short of risk, Alterity is showing investors a trajectory that is supported by science, clinical results, and a clear regulatory pathway. With orphan designation and Fast Track status already in place, the road ahead—though still challenging—now looks more navigable.
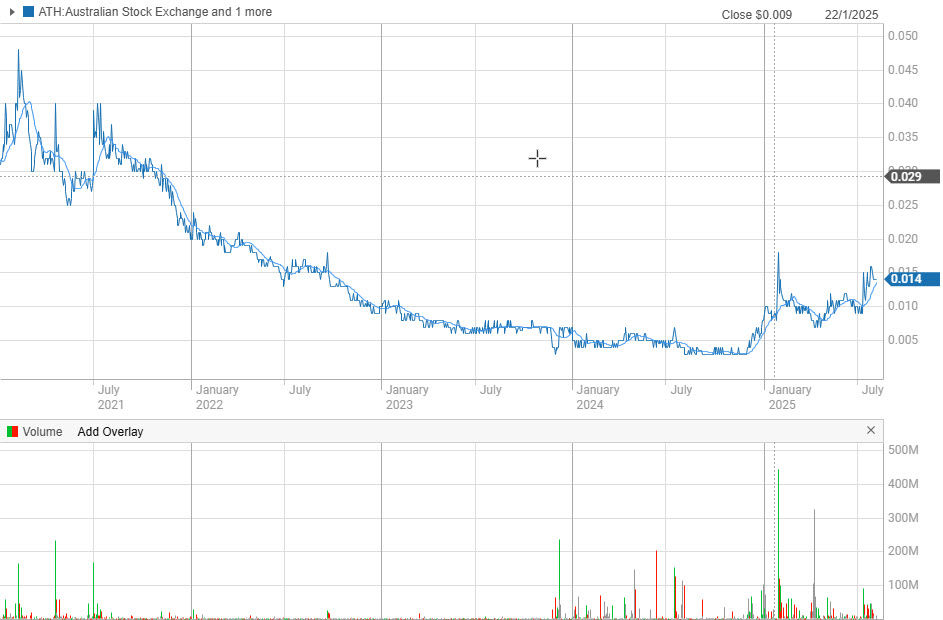
Figure 2: THe share price chart for ATH shows clearly the recent results are consistent with investors confidence in what the company is doing over the last 12 months.
For those watching the neurodegeneration space or seeking differentiated biotech exposure, Alterity is no longer a speculative whisper (Figure 2). It’s a company laying bricks toward something potentially transformative. With a market capitalisation of AUD $128.9M as of writing, this is now a business that could play in the high valuations of the giant biotech companies on the ASx or something in the US markets.
The Samso Way – Seek the Research
Samso encourages investors to dive deeper into the data. Review clinicaltrials.gov (NCT05864365), examine the peer-reviewed studies (e.g., Wenning et al. 2013; Trujillo et al. 2025), and take the time to understand the biomarker strategy Alterity employs. In neurodegenerative disease R&D, nuance matters—and Alterity seems to be getting it right.
Our mission is simple: cut through the noise and spotlight what matters—genuine stories, grounded insights, and real opportunity.
Our content is well-researched and is only created if the team sees a merit in discussing the company or concept. Investors can explore our three core platforms:
There may be numerous paths to success in investing, but the common thread among successful individuals is that they remain committed to making informed decisions. Equip yourself with the right knowledge and tools, and you will be well on your way to achieving your financial goals.
Most importantly, investors need to be absolutely diligent in understanding their own risk-reward tolerance and capabilities. Never bite off more than you can chew. As they say, Rome wasn’t built in a day, and the Great Wall stood because it took centuries to complete.
The Samso Philosophy:
Stay curious. Stay sharp. And remember—digging deeper always uncovers the real value.
In Life, there is no such thing as a Free Lunch.
Happy Investing, and the only four-letter word you need to know is DYOR.
To support our independent nature of our work, please head over to our Support Page and give us a helping hand in any of the ways listed. This is a new initiate for the Samso Platform, and it was always the concept of Samso when we started this journey in 2018.
Disclaimer
The information or opinions provided herein do not constitute investment advice, an offer or solicitation to subscribe for, purchase or sell the investment product(s) mentioned herein. It does not take into consideration, nor have any regard to your specific investment objectives, financial situation, risk profile, tax position and particular, or unique needs and constraints.
Share to Grow: Your Bonus
Samso has just released an eBook: How to Add Value to your Share Portfolio
A lesson on geological models sought by mining companies that gives insight and an understanding of which portfolios are better - and potentially more lucrative – investments. Click here to download this eBook.
If you find this article informative and useful, please help me share the information. I try and write about topics that are interesting and have the potential to be of investment value. It is not easy to find stories that fit those parameters. If you or your organisation see the benefit of what Samso is trying to achieve and have a need to share your journey, please contact me at noel.ong@samso.com.au.
Samso is a trusted platform that equips dedicated investors with up-to-date industry knowledge and insights from top CEOs and thought leaders. By staying informed on business advancements and market trends, investors can enhance their financial decisions through a combination of expert guidance and their own research.





Comments